Following my rotation in Anatomical Pathology, I spent one week on accessioning review, and another three in Chemistry.
BCIT Medical Laboratory Science students get their phlebotomy competencies signed off during a two-week placement in Level 1, so whether you collect blood during the long block practicum is very much dependent on the practicum site. In Kelowna, I had the week of review, and went out 2-3 mornings a week while in Chemistry and Hematology. It’s a useful skill to maintain, as technologists—especially those on off-shifts or at smaller sites—may collect blood throughout their career.
My first week back in Chemistry was spent on the electrophoresis bench. Electrophoresis describes the migration of charged particles in an electric field, and their subsequent separation based on size and charge. The method can be used to examine specific proteins in the blood, urine, and other body fluids.
Are you a BCIT News insider? Sign-up to receive the latest news about BCIT.
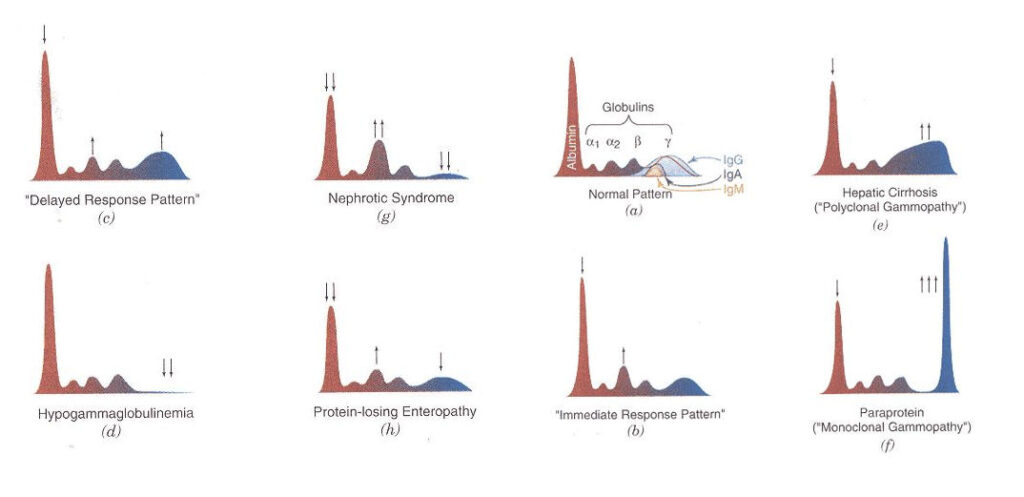
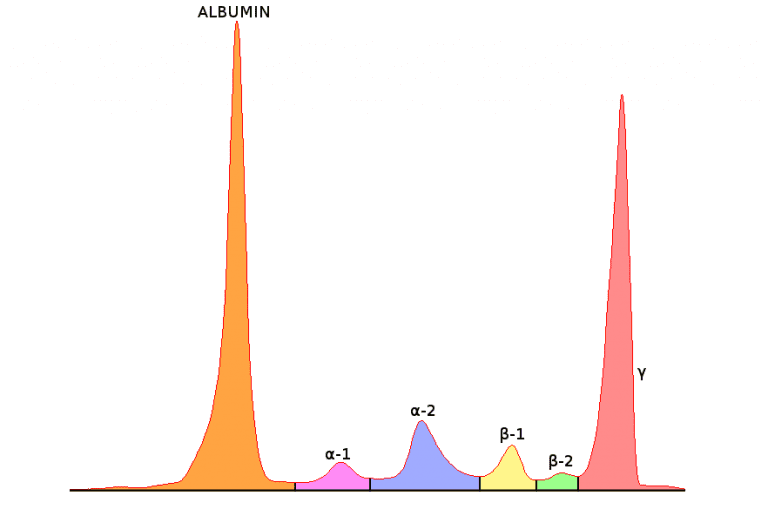
The proteins are separated into five or six fractions and displayed as a curve and corresponding gel pattern. This is a normal serum protein electrophoresis (SPE) pattern—also listed are a few of the proteins associated with each fraction.

Five fractions are shown in this image: albumin, alpha-1 (α1), alpha-2 (α2), beta (β) and gamma (γ). The β region is sometimes divided into β1 and β2, which would account for a sixth fraction.
Once the serum samples are run on the analyzer, a technologist compares the curves with the patients’ previous results and ensures the fractions are appropriately delineated.
If the curve is relatively normal (correct number of peaks and protein values), the analyzer does a pretty good job of identifying each fraction. If abnormal, the curve may be delineated in the wrong location and should be fixed before the result is filed.
There are several reasons a patient’s SPE curve could be abnormal. One pathologic reason—which we covered at BCIT—is a monoclonal gammopathy. Characterized by a sharp peak in the beta-gamma region, monoclonal gammopathies involve increased production of immunoglobulins—or antibodies (such as IgA, IgG, or IgM). As denoted in the normal curve above, antibodies are primarily found in the γ fraction.
Immunoglobulins are secreted by plasma cells—a type of white blood cell in the bone marrow—in response to infection and disease. Typically present in low amounts, conditions such as multiple myeloma and Waldenström’s macroglobulinemia show proliferation of abnormal—or cancerous—plasma cells. More cancer cells lead to more abnormal immunoglobulins, and their build-up negatively affects the formation and function of normal blood cells, the kidneys, and neurologic state—amongst other things.
The presence of a suspected monoclonal can lead to further testing by immunofixation electrophoresis (IFE). IFE classifies the monoclonal based on the type of heavy (IgG, IgA, or IgM) and light (kappa or lambda) chains that make up the antibodies. The procedure was nearly identical to one of our labs at BCIT, so I’m glad to have had a bit of experience in preparing the gel and dispensing the antisera. The stained gel is then sent to a pathologist for diagnosis.
Fortunately, the cause of an abnormal curve is not always pathologic. If the sample is hemolyzed ‘in vitro’ (red blood cells are ruptured outside the body, such as during traumatic blood draw), there will be an increase in free hemoglobin, which migrates to the β1 fraction. The released hemoglobin can also bind to haptoglobin—a protein in the α2 fraction—forming a hemoglobin-haptoglobin complex that blurs the α2 and β separation, and may resemble a malignant peak.
Browse BCIT Health Sciences programs, including the Medical Laboratory Science Diploma.